|
Research Interests
|
|
Created with BioRender.com
|
What are the carrier complexes associated with extracellular miRNA?
miRNA regulate gene expression by base-pairing to complementary mRNA targets while in association with Argonaute, the effector protein of the miRNA mediated silencing complex (miRISC) and eliciting translation repression and/or degradation. Although miRNAs function cell-intrinsically, extracellular miRNAs can be found in circulation, in human blood and cerebrospinal fluid (CSF). Body fluids are rich in RNases. Nevertheless, miRNAs are remarkably stable in these RNase-rich body fluids. The stability of miRNAs in these cell-free body fluids can be attributed to their association with various protective complexes, including extracellular vesicles (EV), Argonaut 2 (AGO2), or high-density lipoproteins. We are interested in exploring the different configurations extracellular miRNAs and their carrier complexes.
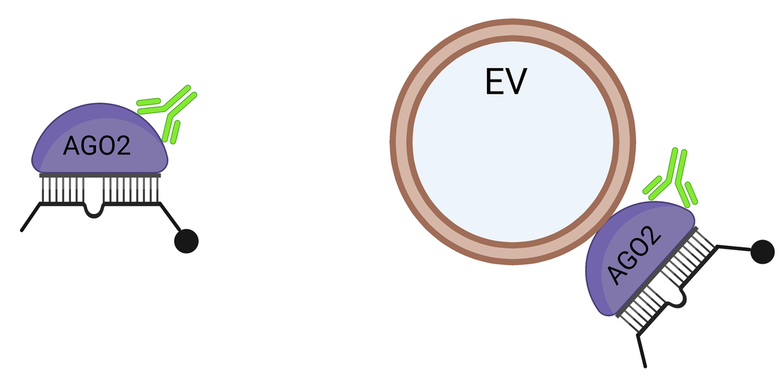
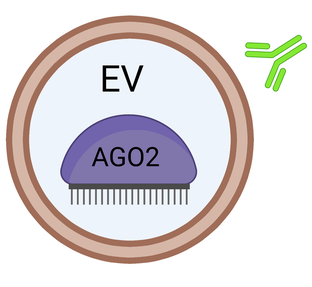
Extracellular miRNA and carrier complex confirmations that are free to interact with AGO2 antibodies and target RNA
Extracellular miRNA and carrier complex confirmations that are NOT free to interact with AGO2 antibodies and target RNA
Created with BioRender.com
miRNAs in human blood plasma and CSF are predominantly contained in functional miRISC/AGO2 complexes that can base-pair with complementary RNA targets in a seed-mediated bona-fide miRISC target recognition manner (Geekiyanage et. al.; PNAS, 2020). This raises the question whether cells/tissues can take-up these functional miRNA complexes from extracellular milieu to regulate their genes. However, the precise origin of these extracellular miRNA-complexes, their export mechanisms, whether they can be delivered to cells and their subsequent functional significances are not understood.
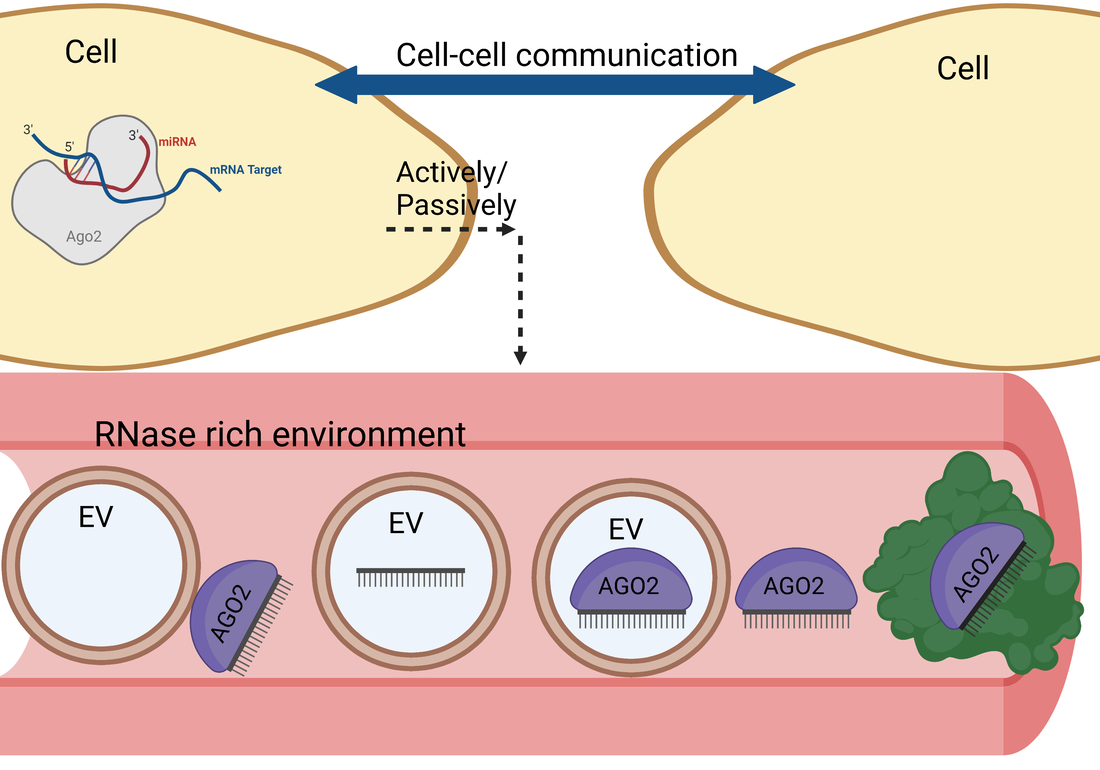
How are intracellular miRNAs exported to the extracellular milieu?
We are interested in
- Identifying the precise origin of these extracellular miRNA-complexes
- Investigating whether miRNAs are released actively or passively from cells
- Determining the miRNA export route
- Determining the genes/proteins/receptors responsible for miRNA export
Can extracellular miRNAs be delivered to the intracellular milieu?
We are interested in
- Investigating whether extracellular miRNAs can be delivered in to the intracellular space
- Determining the circulating miRNA delivery route
- Determining the genes/proteins/receptors responsible for the miRNA delivery
Are extracellular miRNAs delivered to cells/tissues are functional?
Circulating extracellular miRNAs are predominantly associated with miRISC/AGO2 complexes that can engage in seed-mediated base-pairing with a target RNA through a bona-fide miRISC-mediated target recognition (Geekiyanage et. al.; PNAS, 2020). This identification suggests that the endogenous circulating miRNAs can be functional if they are delivered to cells in sufficient amounts. We will use animal models to and in vitro cleavage assays to identify functional capabilities of circulating extracellular miRNAs.
Can the extracellular miRNA carrier complexes be useful as diagnostic biomarkers?
Circulating extracellular miRNAs alone can be used as diagnostic biomarkers (Geekiyanage et. al.; Exp. Neurology, 2012). Furthermore, certain CSF miRNAs that are primarily associated with anti-AGO2 accessible miRISC/AGO2 complexes under normal physiological conditions, are associated with complexes that are unable to interact with AGO2 antibodies under certain neurological conditions (Geekiyanage et. al.; PNAS, 2020). This suggests an unprecedented opportunity to identify a novel class of biomarkers based on the differential assortment of miRNAs into anti-AGO2 antibody assessable or inaccessible miRISC/AGO2 complexes under pathological conditions which may reflect the pathophysiology of the cells of origin, type of stress and cellular viability status. This hypothesis will be tested starting with CSF samples with neurodegenerative disease.
How does intracellular miRNA contribute to Alzheimer’s disease pathology?
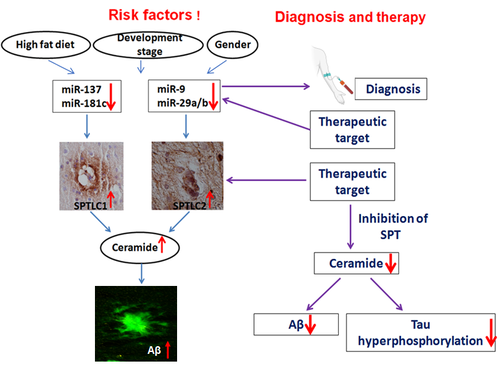
Cell intrinsic miRNAs can directly regulate Serine palmitoyltransferase (SPT), the enzyme responsible for the de novo synthesis of ceramide, and in turn causatively regulated amyloid β (Aβ) levels, a key hallmark of Alzheimer’s disease (Geekiyanage and Chan; J. Neuroscience). Furthermore, an inhibitor of SPT can ameliorate the Aβ burden in a mouse model of Alzheimer’s disease (Geekiyanage et. al.; Neurobio. of Aging). We will continue to address the contribution of miRNA to Aβ production in Alzheimer’s disease.
|
Created with BioRender.com
|
Can viral infections regulate host miRNA?
miRNAs can be utilized to regulate measles virus infectivity and therefore, regulate its oncolytic activity in glioblastoma, breast and ovarian cancers when it is used as a therapeutic intervention (Geekiyanage and Galanis; Mol. Oncology 2016). Host miRNAs can regulate measles virus infectivity and concomitantly, the measles virus infection can also regulate host miRNAs. We will investigate the mechanisms by which viral infections regulate specific host miRNAs.
Technology development: Methodologies to directly detect miRNA binding affinities to their targets
Research geared towards understanding miRNA binding affinities of AGO-miRNA complexes and target RNA has been in the forefront of miRNA research and therefore, identifying the biochemical association/dissociation kinetics becomes vital. Thus far, miRNA affinity-based studies and subsequent Kd value calculations have been conducted based upon RNA-seq data. We are developing a SPR based affinity detection methodologies which will serve as a more direct approach to calculating association kinetics.
Social awareness commitments
Our lab will dedicate efforts to develop an awareness program/ civic engagement platform that will attempt to bridge the scientific community and the general public.